
Sustain vaccination programmes through a healthier vaccine ecosystem in an interconnected Europe
Since the emergence of vaccination, life expectancy has increased between 15-25 years, and further gains are expected. Evidence suggests that the control of infectious diseases through vaccination largely contributed to this increased life expectancy.
Many infectious diseases, which were once commonplace, are increasingly rare because of vaccination. Take smallpox, for example – an infectious disease which killed hundreds of millions of people globally. It is now eradicated thanks to vaccination, and we are on the verge of eradicating polio.
Despite the tremendous progress made, there are major trends of the global public heath context directly impacting the vaccine ecosystem:
Growing/ aging world population which challenges both national health structures and budget
Infectious diseases still being major public health threats, with still circulating infectious agents, like polioviruses or measles, despite existing vaccines and continuous evolution of bacteria and viruses resulting in new emerging diseases (Ebola, Zika…)
Urbanisation and increasing migration and displaced populations from areas of conflicts, which raise new vaccine prevention challenges to be addressed, including for Europe.
Continuing in the path of progress for immunisation is dependent on a healthy vaccine ecosystem.
Over the last decade, the vaccine ecosystem has proven to be vulnerable, with increasing risks of unbalances, the most visible being vaccine shortages.
The global vaccine ecosystem comprises a large variety of actors, including the European actors, both at regional and country levels, notably EU Institutions, Member states governments, healthcare professional associations, vaccine manufacturers, patients’ groups, as illustrated in figure here below.
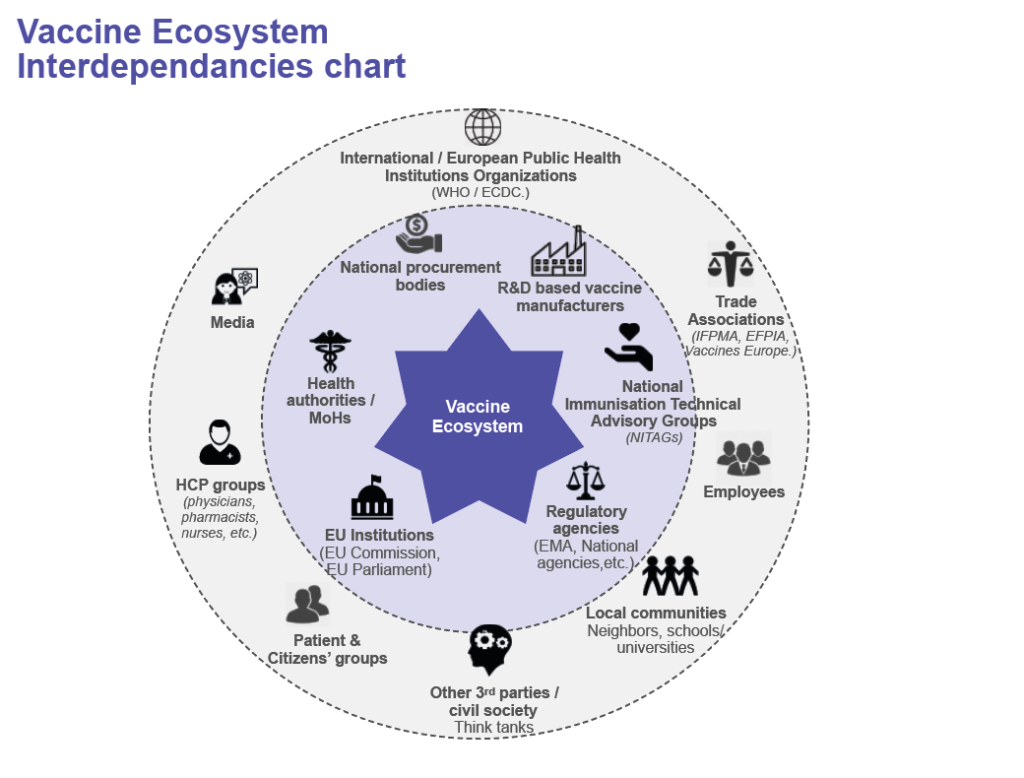
Each of the actors of the ecosystem has its own role and is impacted by the behavior of the others, among others:
- For regulatory agencies, to set guidelines for high quality, safe and efficient vaccines
- For the EU and National public health institutions, to define vaccination policies/programmes according to the epidemiology, existing vaccines and their benefit-risks at population level
- For the procurement agencies, to plan and procure vaccines based on the demand, the immunization programme and the budget defined by the governments
- For the manufacturers, to develop and produce vaccines with the required quality and regulatory standards, and supply countries according to demand signals.
- For the healthcare professionals (HCP), to implement vaccination policies and get target populations vaccinated accordingly
- For the population, to access reliable and accurate information on vaccination they need
Because of these high interdependencies, major impact on one “species” can put at risk the ecosystem balance, with consequences such as reduced access to vaccines/vaccination globally and reduced Research & Development (R&D) investments in new vaccines.
Indeed, still today, we too often see:
- Cost-containment policies and tender processes-based solely on the lowest price criteria resulting from governments and public health partners willingness to minimize vaccine budget.
- Limited visibility on the vaccines demand: knowing that it takes up to 3 years to produce vaccines, a 1-year tender contract does not allow us to anticipate the manufacturing.
- Highly specific national regulations creating delays to vaccine access. For example, to reach a Chinese baby, a vaccine lot manufactured in Europe is tested 3 times and sometimes with different test methods or norms.
These global trends are damaging for the vaccine industry, mainly the R&D based vaccine manufacturers ‘species’ including the ones based in Europe. We produce vaccines that have been researched for worldwide population, investing massively in development of vaccine and large-scale manufacturing biotechnologies as well as manufacturing infrastructures/ facilities. An average of 16% of our income is spent in R&D of new vaccines, complying with highest level of standards of evidence generation (eg. Efficacy Randomised Controlled Trials involving thousands of patients1).
In comparison, the emerging countries vaccine manufacturers produce mainly heritage vaccines for the emerging world that were researched and developed by the R&D-based manufacturers, with a clear focus on high volume and low price. They spend on average 2% of their annual income in R&D, some even benefiting from donors’ or government’s funding for their manufacturing infrastructures.
Over the last decade, the concentration of R&D based manufacturers was observed, with, consequently, shrinkage of R&D investments for new vaccines against new diseases. Only five companies are carrying out 63% of the most urgently needed vaccine R&D projects2.
In addition, decisions for industry to invest in Europe are increasingly challenging as incentives are much higher in other territories like Singapore and the USA. Further loss of vaccine manufacturing capacity and capabilities in Europe would have direct consequences for the European population, with progressive loss of control over its own vaccine supply and over its biodefence.
Europe would then become reliant on imported vaccines supplies and in the event of epidemics or even more worryingly, a pandemic or a biological attack, EU Member States will find themselves competing with non-EU countries for available vaccine supply, risk losing out, as local manufacturers might prioritize their home markets and geopolitical partners. This should raise concerns knowing infectious diseases evolution risks and the antibiotic resistance context.
Invigorating the vaccine ecosystem is critical to sustain vaccination programmes
Fortunately, we see encouraging EU initiatives on vaccination (i.e. Council Recommendation and the Joint Action on Vaccination) that demonstrate the strong commitment from Europe to stay at the forefront of vaccination policies & innovation, and lay the groundwork for a necessary ‘healthier’ vaccine ecosystem. Supply & preparedness to health threats, fight to stop vaccine misinformation dissemination, HCP coalition on vaccination are critical topics that contribute to set up a policy climate supportive of a European-based vaccine industry, with continuous investments in R&D and in manufacturing capacity for a sustainable supply of high-quality vaccines.
In a few months, Germany will take the EU presidency fora a semester, and the European Health Data Space appears to be one of their priorities. Digital transformation of healthcare systems has the potential to boost R&D and innovation in vaccines. Not only it will open a new era in vaccine innovation, but it will also create opportunities to accelerate clinical development of new vaccines or new indications and to reduce the related costs, while assessing the real-world impact of vaccines thanks to the electronic health registries.
Sanofi Pasteur is contributing and committed to engage with all vaccine ecosystem actors to address the key challenges that require coordinated efforts.
Sustainable supply and procurement practices
Sanofi Pasteur has a strong legacy in vaccines and produce vaccines against 7 bacterial diseases and 8 viral diseases. More than 1 billion doses of vaccines are produced each year in 12 sites, in France, North & South America, and Asia.
We all concur on the fact that poor forecasting, inappropriate procurement mechanisms and increasing regulatory barriers are impacting ability to supply vaccines to the population.
At Sanofi Pasteur, we have conducted our own assessment of the roots causes that impact our capacity to supply our vaccines through a specific company initiative to improve supply security and we have action plans in place to optimize our Industrial affairs processes accordingly. In parallel, we actively contributes to the existing collaborative work within the trade associations at global and EU levels (IFPMA & VE), with regular interfaces with WHO on this topic. Optimising public procurement practices and better demand anticipation is also central in our discussions with Health Authorities at National levels.
Continuous investment in vaccine Research, development & Innovation
As R&D remains Sanofi Pasteur’s DNA, we are investing more than 500 million euros each year, with five R&D sites (Europe and North America) and employees representing 15% of the overall Sanofi Pasteur’s staff.
However, due to drastic evolution of regulatory standards and requirements for pre-clinical and clinical trials, the cost of development (R&D, global licenses) and industrial equipment for a new vaccine is estimated between 0,5 and 1 billion Euros. As a result, the private sector can no longer develop new vaccines at risk, without any visibility on a possible return on investment.
Interesting new solutions approaches have emerged as either public-private partnerships.
These are new sources of value sharing, coordination and cooperation for the world, as for instance for the usage of electronic health data. Sanofi Pasteur is committed to R&D open innovation, notably via Innovative Medicine Initiative (IMI) projects (eg. Antimicrobial resistance, healthy ageing, pertussis) in Europe and contribute to the ongoing discussions on the new Horizon Europe 2021-2027 framework.
Use of electronic medical records in new vaccine development
The development of a new vaccine classically requires from 10 to 15 years with massive investment ranging from $200 million to $1 billion (including construction of manufacturing facilities).
Convinced by the fact that digital health data opens a new era in vaccine, Sanofi Pasteur is supporting the Northern California Kaiser Permanente (NCKP) vaccines research unit in the conduct of an unprecedented, innovative real-world evidence clinical study to evaluate the relative effectiveness of different flu vaccines available in the US.
The data used are generated during routine clinical practice within the NCKP healthcare system in the US, using their integrated digitalized patient record system enabling the tracking of influenza events, complications in outpatient and inpatient facilities and vaccination status. The novel approach of this pragmatic study allows the prospective inclusion and cluster randomization of a total of 1.6 million vaccinees over two years – which would, have never been possible in the traditional clinical trial setting.
Similarly, we have just started a similar pragmatic randomized controlled database trial in partnership with the Finnish Institute of Health and Welfare (THL). This study possible in a European country is one example that demonstrates the power that integrated electronic health registries can have on vaccine evaluations, allowing better understand the performance of vaccines in a real-world environment, helping to demonstrate the value of a vaccine, and providing insights for future vaccines development.
Conclusion
As recently mentioned by Professor Panos Kanavos ‘In order for the vaccine ecosystem to be “healthy”, all relevant stakeholders should be taken into account’3…, including the vaccine industry.
Moving forward, we should maintain dialogue and jointly seek common solutions for:
a more balanced and healthier vaccine ecosystem, in order to tape the full potential that vaccination has both on the sustainability of the European healthcare systems, and on releasing pressure on its health care resources.
a favorable environment for vaccine industry investments in R&D and manufacturing in the EU countries, that provide similar incentives than other attractive regions
an interconnected Europe that facilitates patient electronic medical data collection, including vaccination records, and enables interoperability of health information systems to connect data, for the benefits of public health and vaccines innovation.
This would contribute to a healthier Europe, where ‘no one should die or suffer from a vaccine-preventable disease’ and to reduce cross border health threats.
1 – https://clinicaltrials.gov/ct2/show/NCT01427309
2 – 2018 Stat+ – Access to Medicine Foundation report https://www.accesstomedicineindex.org/publications/2018-access-to-medicine-index
3 – Professor Panos Kanavos (associate professor LSE) during the European Health Policy Forum Gastein https://www.euractiv.com/section/health-consumers/special_report/a-healthy-vaccines-ecosystem/1388050/
 |
 |
 |
| Corinne Bardone, Pharm D Head of Global Vaccines Public Affairs, for Polio, Pertussis and Hib containing Vaccines, Sanofi Pasteur |
Christina Klein Manager, Global Vaccine Public Affairs, Sanofi Pasteur |
Florence Baron-Papillon, Pharm D Head of Public Affairs Europe for Vaccines, Sanofi Pasteur |



